Bnt162b2 Studie, Humoral Response To The Pfizer Bnt162b2 Vaccine In Patients Undergoing Maintenance Hemodialysis American Society Of Nephrology
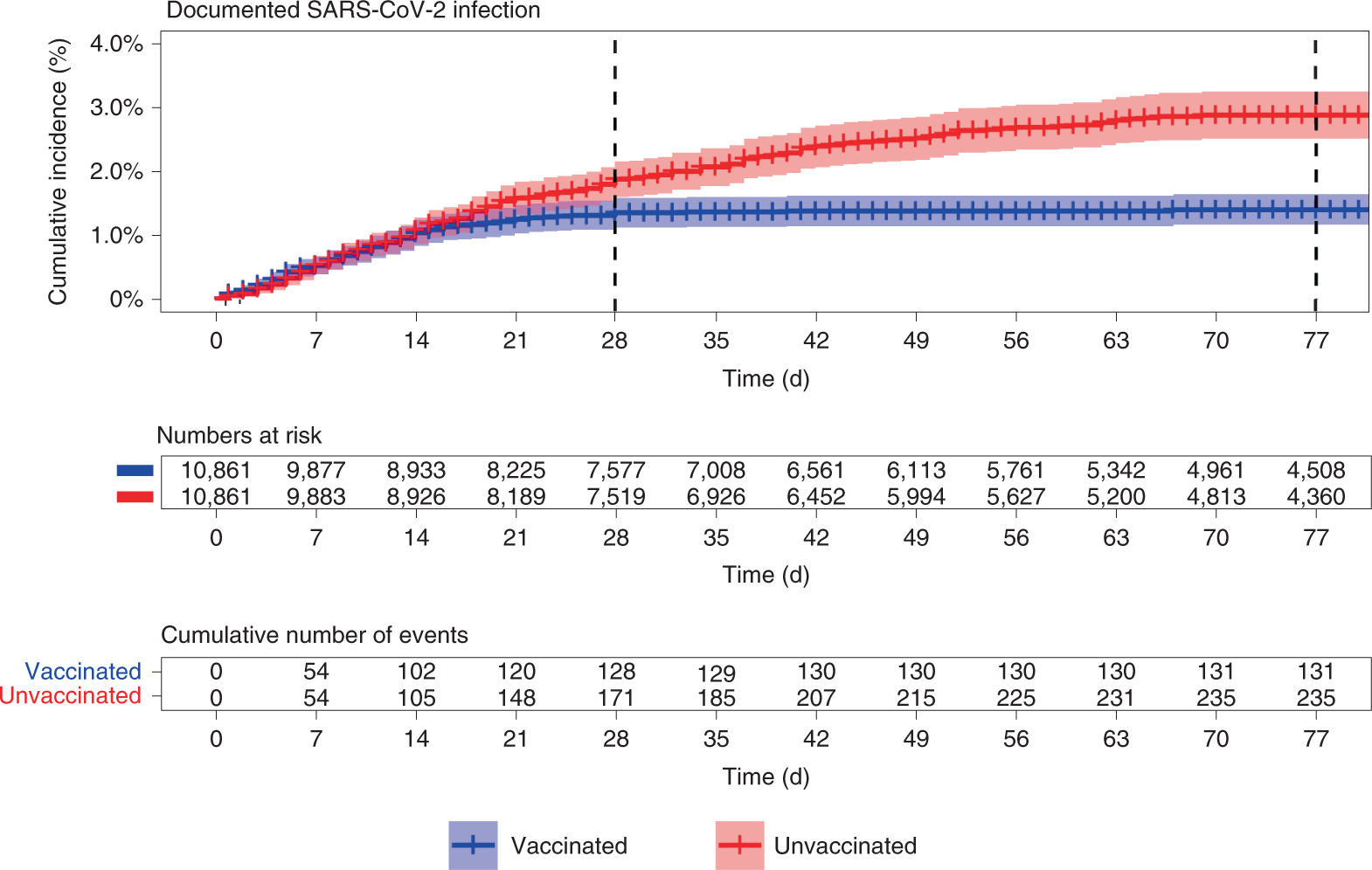
With the evidence of waning immunity of the BNT162b2 vaccine a national third dose vaccination campaign was initiated in Israel during August 2021. To further describe potential homologous and heterologous protection against emerging SARS-CoV-2 VOCs a new cohort of participants will be enrolled who are COVID-19 vaccine-naïve ie BNT162b2-naïve and have not experienced COVID-19.
Bnt162b2 Induces Sars Cov 2 Neutralising Antibodies And T Cells In Humans Medrxiv
Overall 2260 adolescents 12 to 15 years of age received injections.
Bnt162b2 studie. The vaccines BNT162a1 BNT162b1 BNT162b2 and BNT162c2 will be administered using a PrimeBoost PB regimen. Vaccine effectiveness VE studies have not differentiated the impact of Delta from potential waning immunity on recent observed reductions in effectiveness against SARS-CoV-2 infections. This study investigated persistence of BNT162b2 Pfizer-BioNTech vaccine effectiveness against infection and disease in Qatar where the Beta and Delta variants have dominated incidence and PCR testing is done at a mass scale.
METHODS A matched test-negative case-control. A further subset of Phase 3 participants will receive a third lower dose of BNT162b2 at 5 or 10 µg. The vaccine BNT162c2 will also be administered using a Single dose SD regimen.
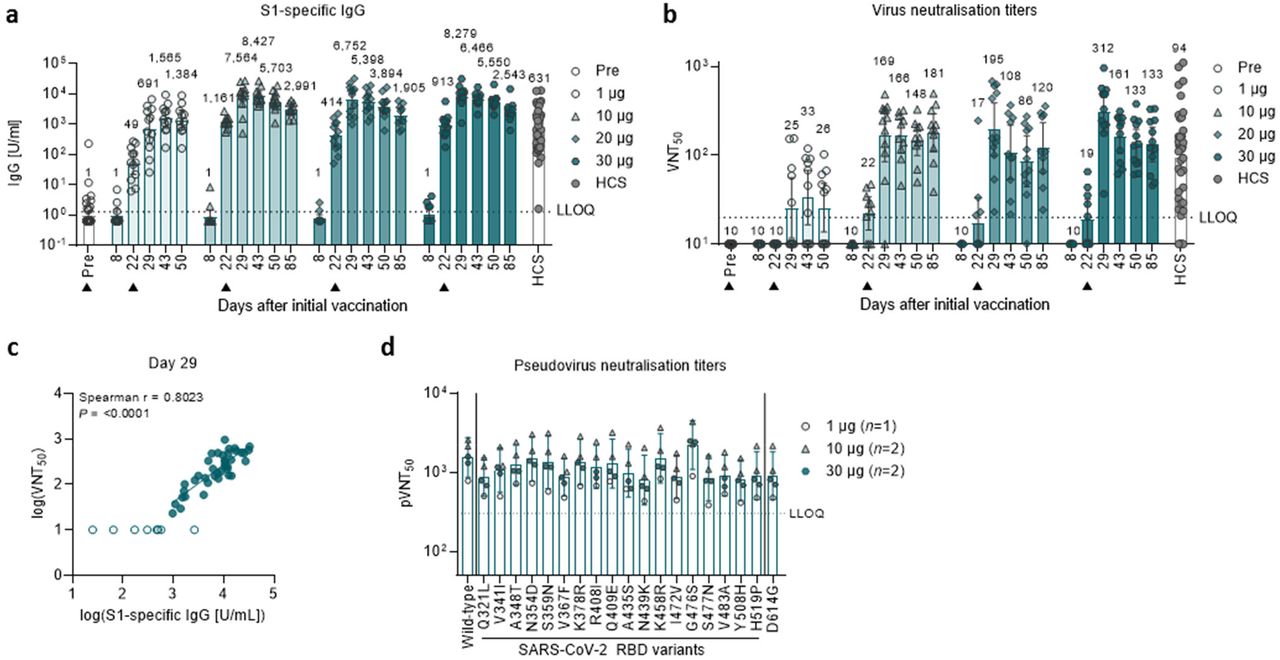
A large population-based study found that allergic reactions to vaccines generally occur at a rate of 131 95 confidence interval 090184 cases per million vaccine doses with no fatalities reported McNeil et al 2016. As effectiveness of BNT162b2 was studied in patients more than 7 days after the second dose in most clinical studies. They have enabled us to advance BNT162b2 into Phase 3 evaluation said Ugur Sahin MD CEO and.
A systematic literature search with no language restriction was performed in electronic databases to identify eligible observational studies which reported the adjusted effectiveness of the BNT162b2 mRNA vaccine to prevent RT-PCR confirmed COVID-19. 18-55 years of age. The Applicant refers to that as they are not considered necessary according to the WHO guideline WHO 2005.
A single dose of BNT162b2 vaccine showed vaccine effectiveness of 70 95 CI 55-85 21 days after first dose and 85 74-96 7 days after two doses in the study population. The data we have shared today include the characterization of our lead candidate BNT162b2 as well as key animal studies that were the basis for our clinical programs. No safety pharmacology studies were conducted with BNT162b2.
Three additional cohorts aged from 18 to 85 years receiving BNT162b2 only. We evaluated overall and variant-specific effectiveness of BNT162b2 against SARS-CoV-2 infections and COVID-19-related hospitalizations by time. A relationship between neutralization level after SARS-CoV-2 vaccination and protection against COVID-19 has been demonstrated by several studies.
Leveraging data from Maccabi Healthcare Services we conducted a preliminary retrospective study aimed at evaluating initial short-term effectiveness of a. In addition no findings on vital organ functions have been recorded in the repeat dose toxicology studies. BNT162b2 is a lipid nanoparticle-formulated nucleoside-modified RNA vaccine that encodes a prefusion stabilized membrane-anchored SARS-CoV-2 full-length spike protein.
The primary end points were efficacy of the vaccine against laboratory-confirmed Covid-19 and safety. The BNT162b2 vaccine consists of two doses 30 μg 03 mL each administered intramuscularly 3 weeks apart. Thus the absence of safety pharmacology studies is endorsed by the.
We show that BNT162b2 was 65 effective in preventing infections following exposures and 83 effective in preventing never-symptomatic infectious N-gene Ct value. 4 As such the height of the humoral response after vaccination which correlates with neutralizing antibody titers 5 might be clinically relevant. As has been found in other age groups BNT162b2 had a favorable safety and side-effect profile with mainly transient mild-to-moderate reactogenicity predominantly injection-site pain in 79 to 86 of participants fatigue in 60 to 66 and headache in 55 to 65.
BNT162b2 Phase 1 Studies. BACKGROUND Waning of vaccine protection against SARS-CoV-2 infection or COVID-19 disease is a concern. Included were patients who received two doses of BNT162b2 had a PCR-confirmed diagnosis of severe acute respiratory syndrome coronavirus 2 SARS-CoV-2 infection and were hospitalized in a COVID-19-dedicated unit.
Other countries have announced their intention to administer a booster shot as well. This paper aims to summarize through meta-analyses the overall vaccine effectiveness of the BNT162b2 mRNA vaccine from observational studies. 18-55 and 65-85 years of age.
1131 received BNT162b2 and 1129 received placebo. Safety of the BNT162b2 mRNA Covid-19 Vaccine Among more than 17 million persons BNT162b2 vaccination was associated with increased risks of myocarditis risk ratio 324 lymphadenopathy append.

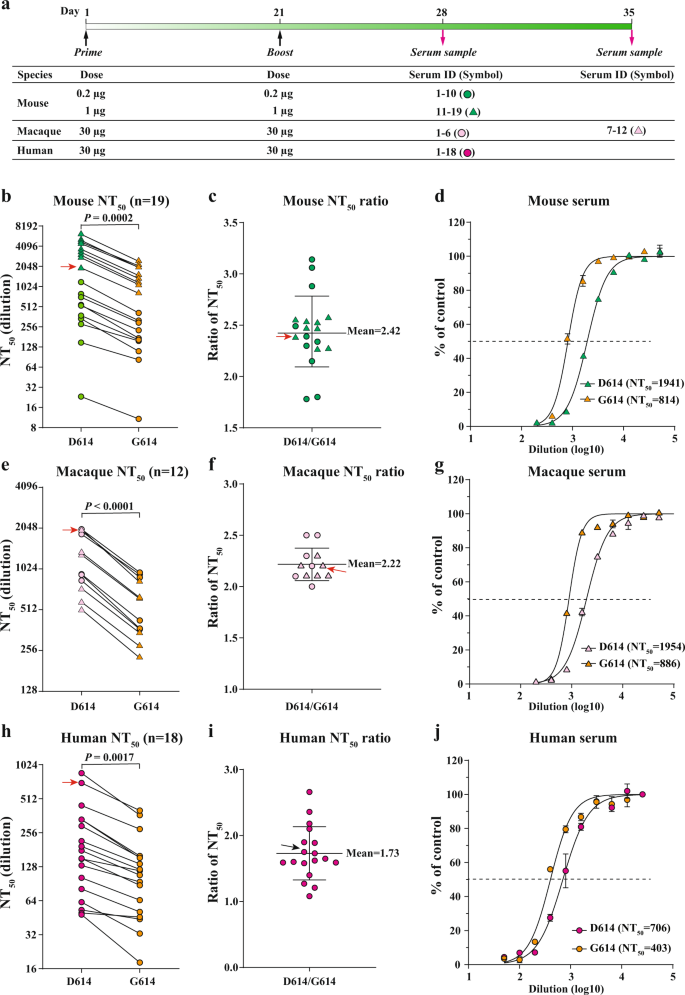
The Effect Of Sars Cov 2 D614g Mutation On Bnt162b2 Vaccine Elicited Neutralization Npj Vaccines
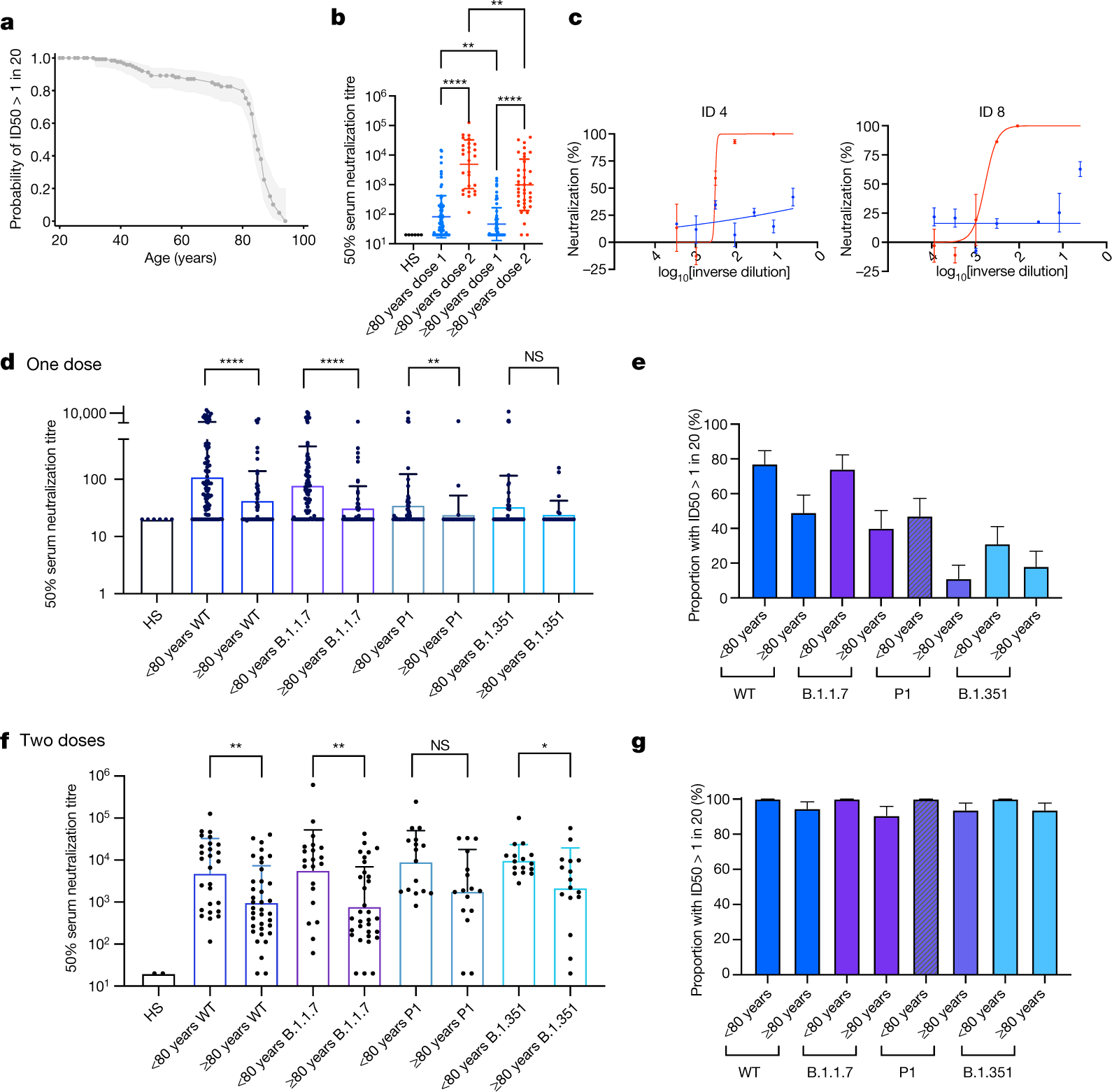
Age Related Immune Response Heterogeneity To Sars Cov 2 Vaccine Bnt162b2 Nature

Humoral Response To The Pfizer Bnt162b2 Vaccine In Patients Undergoing Maintenance Hemodialysis American Society Of Nephrology

Spike Antibody Waning After Second Dose Of Bnt162b2 Or Chadox1 The Lancet

Bnt162b2 Vaccine Breakthrough Clinical Characteristics Of 152 Fully Vaccinated Hospitalized Covid 19 Patients In Israel Clinical Microbiology And Infection

Neutralizing Response Against Variants After Sars Cov 2 Infection And One Dose Of Bnt162b2 Nejm

Safety And Immunogenicity Of One Versus Two Doses Of The Covid 19 Vaccine Bnt162b2 For Patients With Cancer Interim Analysis Of A Prospective Observational Study The Lancet Oncology
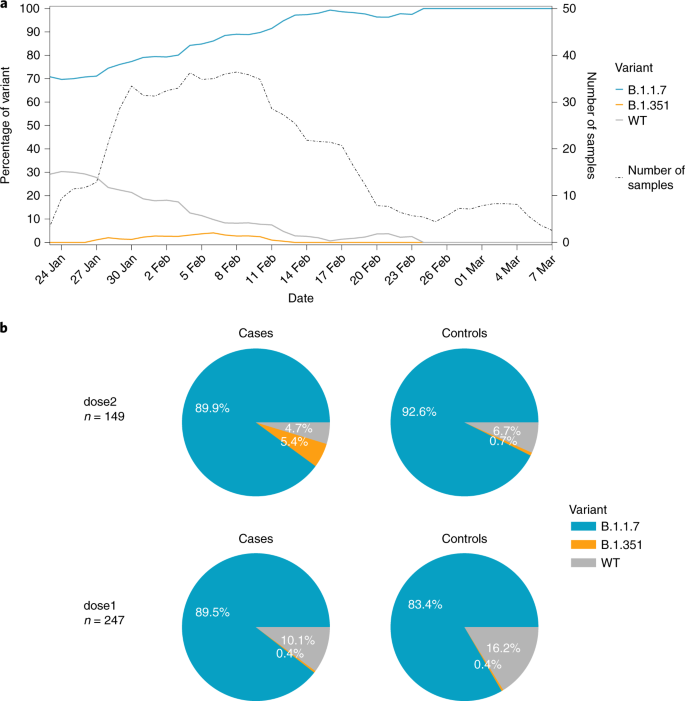
Evidence For Increased Breakthrough Rates Of Sars Cov 2 Variants Of Concern In Bnt162b2 Mrna Vaccinated Individuals Nature Medicine

Interim Estimates Of Vaccine Effectiveness Of Bnt162b2 And Mrna 1273 Covid 19 Vaccines In Preventing Sars Cov 2 Infection Among Health Care Personnel First Responders And Other Essential And Frontline Workers Eight U S Locations December
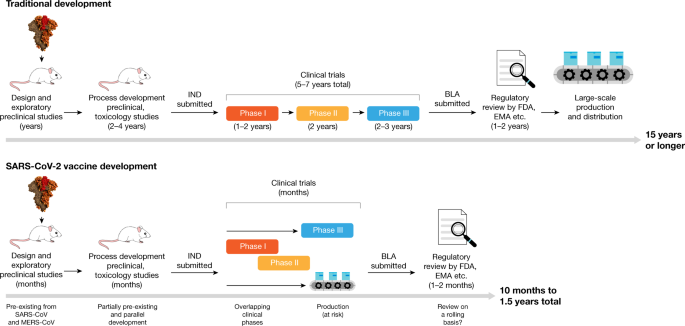
Sars Cov 2 Vaccines In Development Nature
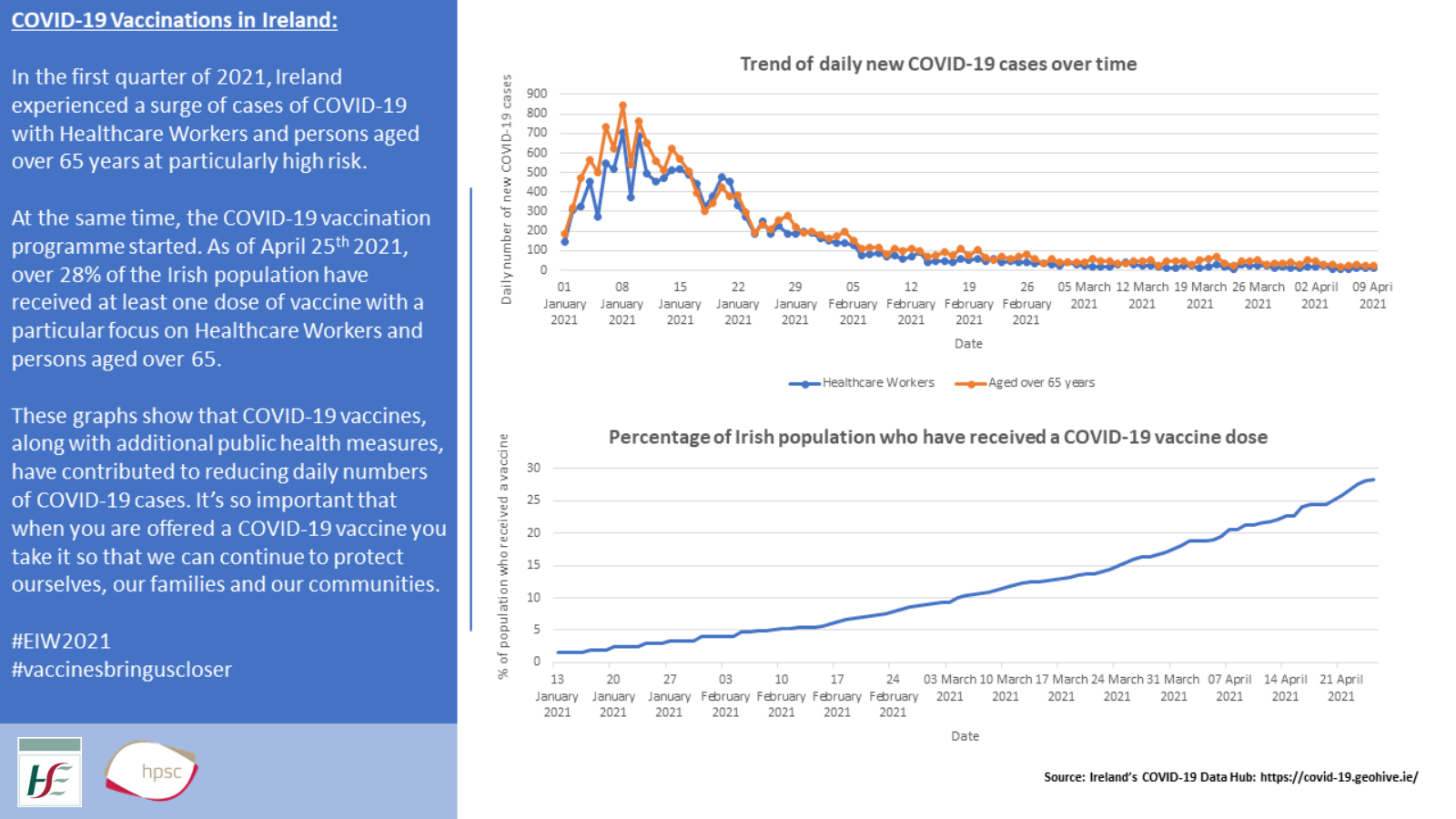
Covid 19 Vaccine Studies Hse Ie
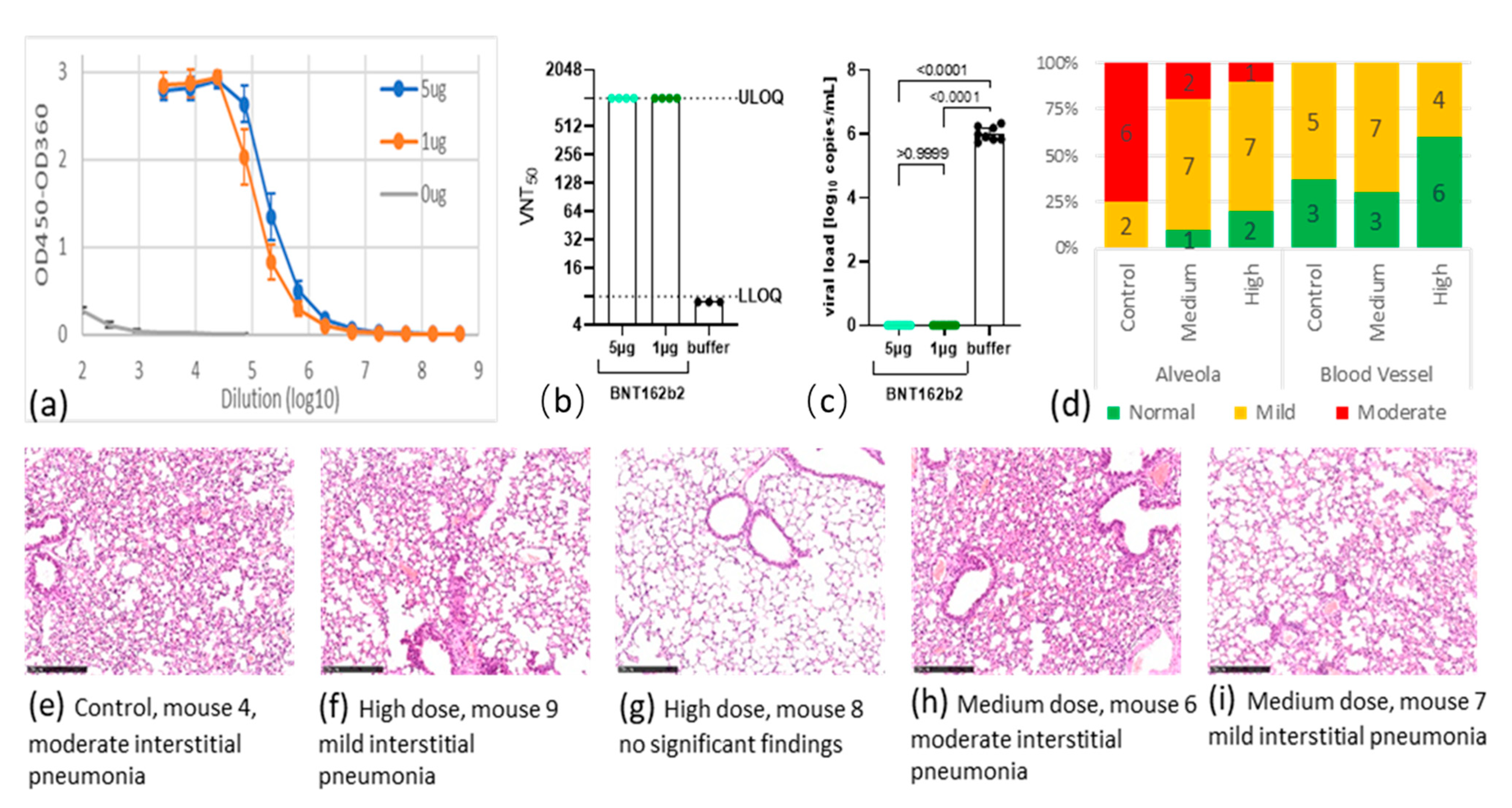
Vaccines Free Full Text Bnt162b2 Vaccine Encoding The Sars Cov 2 P2 S Protects Transgenic Hace2 Mice Against Covid 19 Html

Safety Immunogenicity And Efficacy Of The Bnt162b2 Covid 19 Vaccine In Adolescents Nejm

Safety And Efficacy Of The Bnt162b2 Mrna Covid 19 Vaccine Nejm
Phase Iii Studie Zu Corona Vakzine Bnt162b2 Jetzt Publiziert

Safety And Efficacy Of The Bnt162b2 Mrna Covid 19 Vaccine Nejm
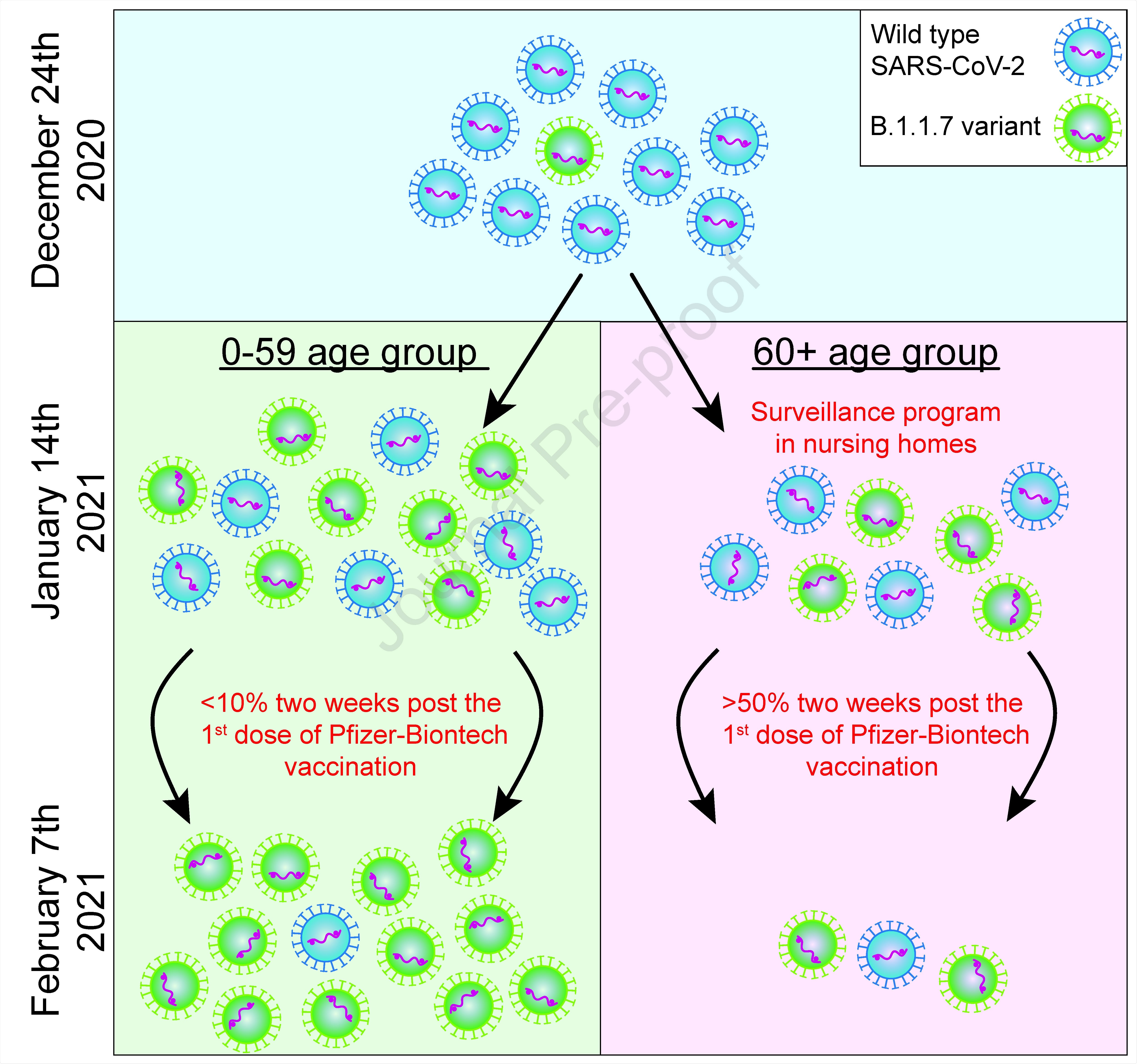
Pfizer Biontech Bnt162b2 Vaccine Successfully Curbs Covid 19 In Israel

Safety And Efficacy Of The Bnt162b2 Mrna Covid 19 Vaccine Nejm