Bnt162b2 | Bnt162b2 Vaccine Breakthrough Clinical Characteristics Of 152 Fully Vaccinated Hospitalized Covid 19 Patients In Israel Clinical Microbiology And Infection
Prevention of Coronavirus Disease 2019 COVID-19 caused by SARS-CoV-2. BNT162b2 is a lipid nanoparticle-formulated nucleoside-modified RNA vaccine that encodes a prefusion stabilized membrane-anchored SARS-CoV-2 full-length spike protein.

A Prefusion Sars Cov 2 Spike Rna Vaccine Is Highly Immunogenic And Prevents Lung Infection In Non Human Primates Biorxiv
DOSE LEVEL and REGIMEN 30 µg 2 doses.

Bnt162b2. Learn about safety data efficacy and clinical trial demographics. Investors were already optimistic about prospects for it but after comparing data the companies decided instead to pursue another candidate BNT162b2 Sahin said. The BNT162b2 mRNA COVID-19 vaccine is safe and well-tolerated in patients with mast cell disorders.
Mast cell MC disorders MCDs are characterized by the proliferation and accumulation of MCs in different tissues including skin and bone marrow andor. The primary end points were efficacy of the vaccine against laboratory-confirmed Covid-19 and safety. These patients can be immunized with this vaccine with no need for specific measures.
In this study in a nationwide mass vaccination setting the BNT162b2 vaccine was not associated with an elevated risk of most of the adverse events examined. The Pfizer-BioNTech COVID-19 vaccine is an mRNA vaccine that requires 2 shots 21 days apart. Safety of the BNT162b2 mRNA Covid-19 Vaccine in a Nationwide Setting N Engl J Med.
The BioNTech technology for the BNT162b2 vaccine is based on use of nucleoside-modified mRNA modRNA which encodes a mutated form of the full-length spike protein found on the surface of the SARS-CoV-2 virus triggering an immune response against infection by the virus protein. Online ahead of print. Individuals 16 years of age and older.
The vaccine was associated with an excess risk of myocarditis 1 to 5 events per 100000 persons. The company also said that it expects roughly 335 billion in sales from BNT162b2 in 2021 which is much higher than the 26 billion Pfizer had projected in its first-quarter earnings release. Pan-Sarbecovirus Neutralizing Antibodies in BNT162b2-Immunized SARS-CoV-1 Surviv.

Uk Authorises World S First Covid 19 Vaccine Pfizer Biontech S Bnt162b2

Bnt162b2 Vaccine Breakthrough Clinical Characteristics Of 152 Fully Vaccinated Hospitalized Covid 19 Patients In Israel Clinical Microbiology And Infection

Pfizer And Biontech To Supply Eu With Up To 200m Doses Of Bnt162b2 Against Covid 19 Pharmashots
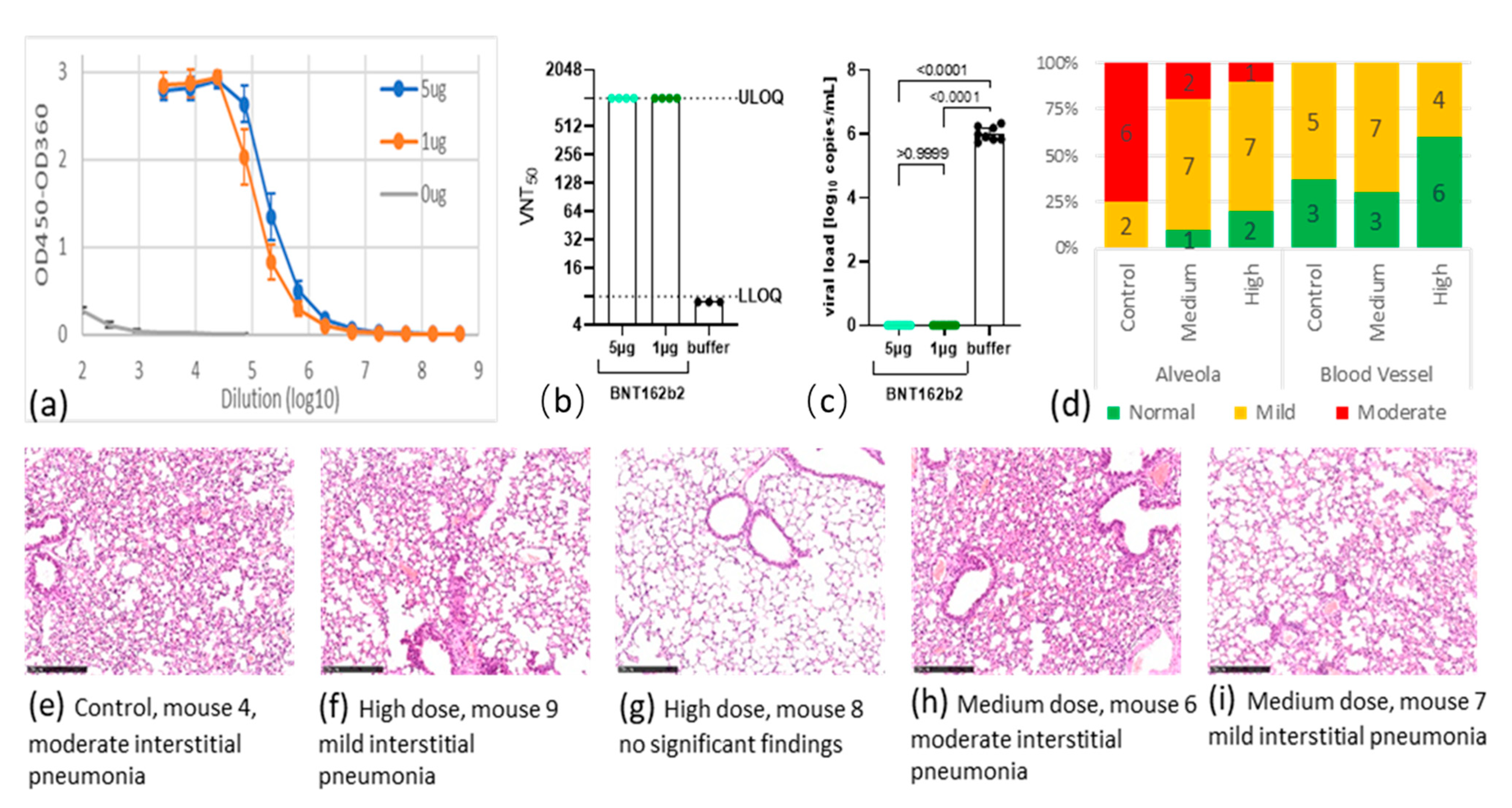
Vaccines Free Full Text Bnt162b2 Vaccine Encoding The Sars Cov 2 P2 S Protects Transgenic Hace2 Mice Against Covid 19 Html
Biontech Vaccine Mass Production In China Close To Ready May Get Go Ahead Before July Global Times
Studienlage Corona Impfstoff Bnt162b2 Gelbe Liste

Key Committee Endorses Pfizer S Covid 19 Vaccine Clearing Way For Fda
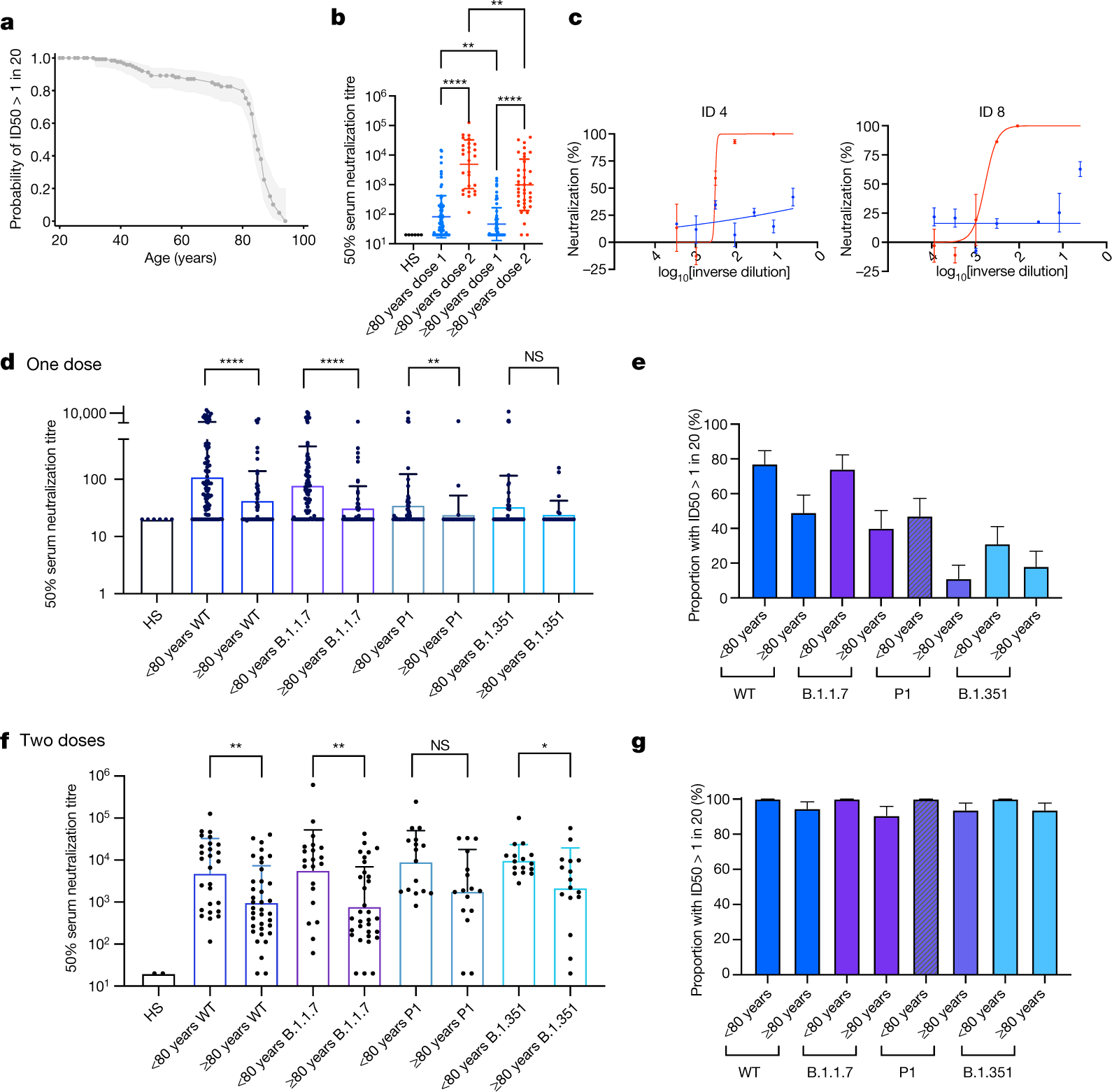
Age Related Immune Response Heterogeneity To Sars Cov 2 Vaccine Bnt162b2 Nature
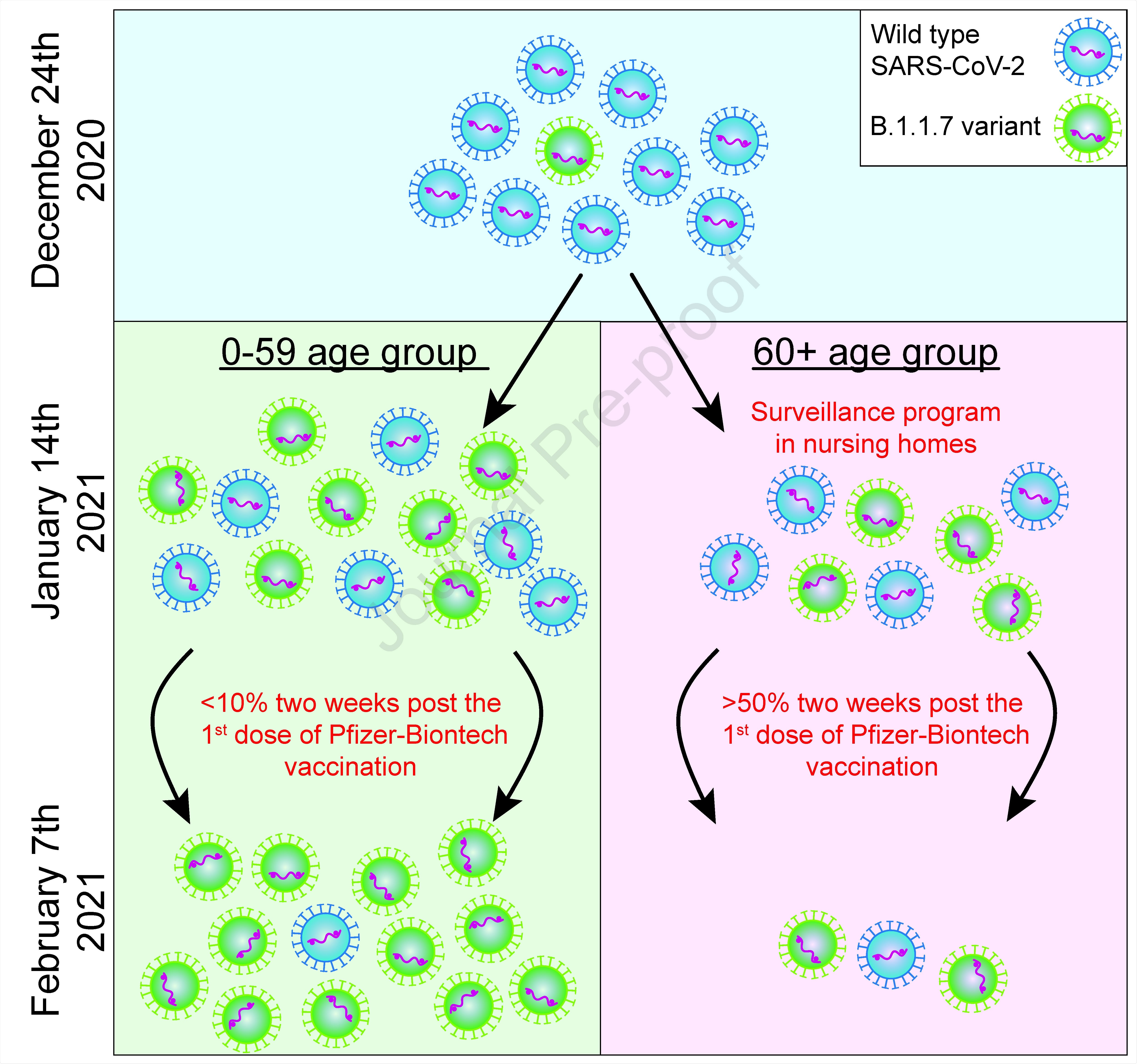
Pfizer Biontech Bnt162b2 Vaccine Successfully Curbs Covid 19 In Israel
Fda Grants Emergency Use Authorization For Pfizer S Covid 19 Vaccine

Safety And Efficacy Of The Bnt162b2 Mrna Covid 19 Vaccine Ers European Respiratory Society

Medical Product Alert N 2 2021 Falsified Covid 19 Vaccine Bnt162b2
Pfizer And Biontech To Supply The Eu With 200 Million Doses Of Bnt162b2

Safety And Efficacy Of The Bnt162b2 Mrna Covid 19 Vaccine Nejm

Antibody Response To First Bnt162b2 Dose In Previously Sars Cov 2 Infected Individuals The Lancet
Bnt162b2 Covid 19 Vaccine Is 95 Percent Effective Finds Final Analysis
Data From Israel On The Bnt162b2 Pfizer Mrna Covid 19 Vaccine Rebel Em Emergency Medicine Blog
Pfizer Biontech Win Eu Authorisation For Covid 19 Vaccine Comirnaty Pharmatimes
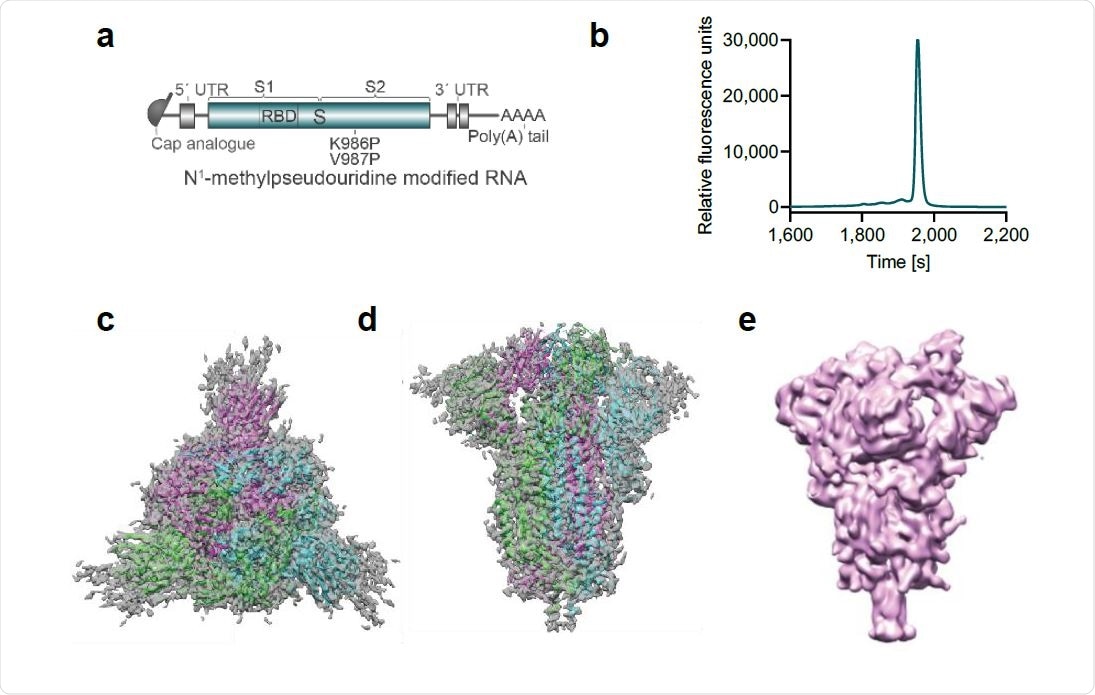
Pfizer Biontech Covid 19 Vaccine Candidate Shows Promise In Preclinical Study
