Bnt162b2sa : Https Kce Fgov Be Sites Default Files Atoms Files Third 20covid 19 20vaccination Report Dutch Pdf
BNT162b2 is a candidate vaccine being developed by BioNTechPfizer. Or BNT162b2SA 1 2 9.

Pfizer Biontech S Covid 19 Vaccine Not To Require A Booster In 6 12 Months
Estimated Enrollment O Allocation.

Bnt162b2sa. 6 BNT162b2 n 22085 Placebo n22080 Inclusion criteria. Registre des essais cliniques. PfizerBioNTech has two booster strategiesa third Comirnaty dose or its BNT162b2SA shot which is directed to B1351 the VOC first detected in South Africa.
Multicenter Multinational Follow-up duration months. Titelmasterformat durch Klicken bearbeiten Strong Order Book Growth in Q1 10 18 billion doses contracted for 2021 Selected Regions Current. Male or female participants between the ages of 18 and 55 years inclusive at enrollment.
For 7 days after dose 1 and dose 2. It is an mRNA type of candidate vaccine based on the RNA platform. They will receive BNT162b2SA given as a 2-dose series separated by 21 days.
The PfizerBioNTech COVID-19 vaccine BNT162b2 is an mRNA vaccine encoding a P2 mutant spike protein PS 2 and formulated as an RNAlipid nanoparticle of. Participants 12 yo or. To further describe potential homologous and heterologous protection against emerging SARS-CoV-2 VOCs a new cohort of participants will be enrolled who are COVID-19.
They will receive BNT162b2SA given as a 2-dose series separated by 21 days. In the Phase 3 portion of this clinical trial adolescents 12-15 years. Newly enrolled participants enrolled to receive 2 doses of BNT162b2SA.
BNT162b2SA Phase O Phase 2 Phase 3 Go to COVID-19 Study Design Study Type O. In a release Monday both healthcare systems announced it joined two phase 3 clinical trials to test Pfizers new BNT162b2SA vaccine which mimics the genetic code of. BIOmarker-styrede behandlingsbeslutninger i psoriasis- og reumatoid arthritis.
By evaluating BNT162b2SA as a prototype vaccine the companies aim to inform the development of an efficient regulatory pathway for testing future modified mRNA. A Trial Investigating the Safety and Effects of Four BNT162 Vaccines Against COVID-2019 in Healthy and Immunocompromised Adults - Full Text View. In participants who receive BNT162b2SA given as 1 or 2 doses percentage of participants reporting systemic events Time Frame.
Some BNT162b2SA-vaccinated participants will also receive a second dose of the modified vaccine to investigate if it would be needed to reach noninferior.

Riny ヅ On Twitter Alle Vaccines Voor Covid 19 Zijn In Een Experimentele Fase Voorbeeld Hier Weergegeven Is Pfizer Biontech Https T Co Lgzgglrbkt Uit Ethisch Gemak Hebben De Mensen Die Het Placebo Kregen Ook Een Echt
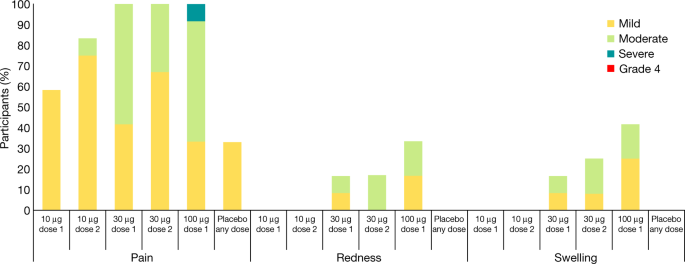
Phase I Ii Study Of Covid 19 Rna Vaccine Bnt162b1 In Adults Nature
Https Kce Fgov Be Sites Default Files Atoms Files Third 20covid 19 20vaccination Report Dutch Pdf
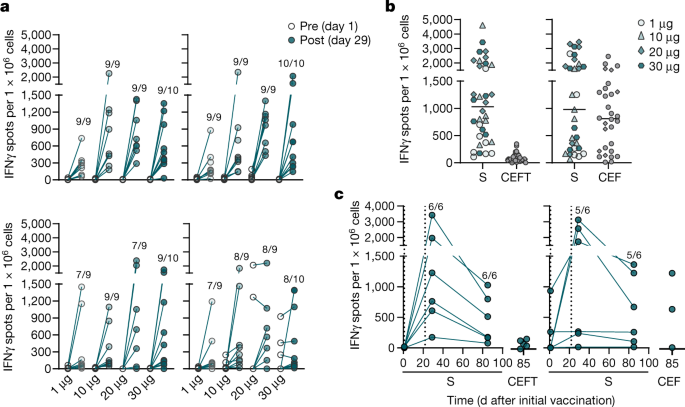
Bnt162b2 Vaccine Induces Neutralizing Antibodies And Poly Specific T Cells In Humans Nature
Https Kce Fgov Be Sites Default Files Atoms Files Third 20covid 19 20vaccination Report Dutch Pdf
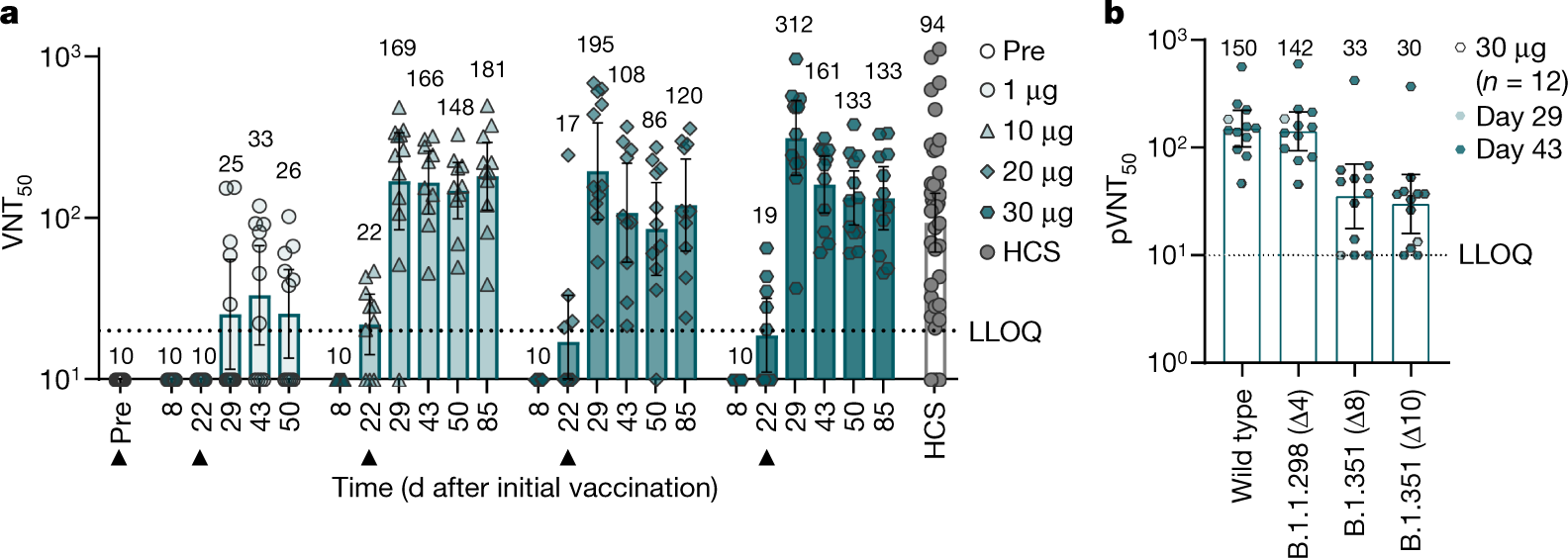
Bnt162b2 Vaccine Induces Neutralizing Antibodies And Poly Specific T Cells In Humans Nature
Https Kce Fgov Be Sites Default Files Atoms Files Third 20covid 19 20vaccination Report Dutch Pdf

Covid Hospitalisation Spike In The Us A Trigger For Future Mrna Deployment

Ur Medicine Rrh Take Part In Trial For New Pfizer Vaccine Targeted At Variant Whec Com

Study To Describe The Safety Tolerability Immunogenicity And Efficacy Of Rna Vaccine Candidates Against Covid 19 In Healthy Individuals Full Text View Clinicaltrials Gov

University Of California Health Covid 19 Clinical Trials California
Https Kce Fgov Be Sites Default Files Atoms Files Third 20covid 19 20vaccination Report Dutch Pdf

Study To Describe The Safety Tolerability Immunogenicity And Efficacy Of Rna Vaccine Candidates Against Covid 19 In Healthy Individuals Full Text View Clinicaltrials Gov

Ur Medicine Rrh Take Part In Trial For New Pfizer Vaccine Targeted At Variant Whec Com

Study To Describe The Safety Tolerability Immunogenicity And Efficacy Of Rna Vaccine Candidates Against Covid 19 In Healthy Individuals Full Text View Clinicaltrials Gov
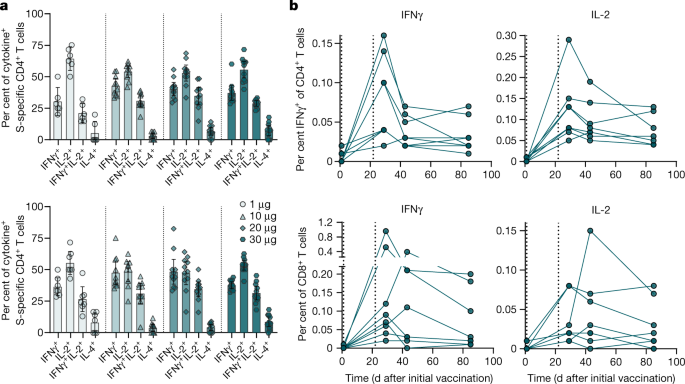
Bnt162b2 Vaccine Induces Neutralizing Antibodies And Poly Specific T Cells In Humans Nature



